Czech scientists, in cooperation with Chinese colleagues, have been the first to show the structure and determine mobility of ionic sodium hydrates, clusters made of water molecules and sodium atoms, involved in a number of significant physical, chemical and biological processes. Thanks to a special method, they confirmed that the mobility of these miniature objects, which affects their effectiveness, is related to the internal arrangement. The study was published recently in the journal Nature.
Pavel Jelínek from the Institute of Physics of the Academy of Sciences of the Czech Republic and the Regional Centre of Advanced Technologies and Materials of Palacký University Olomouc took part in the work. According to him, the team managed to fill one of the blank spaces in chemistry, biology and physics. Hydration of ions on surfaces is of great importance, for example, in corrosion, electrochemistry, and transport of ions in living organisms. Without ion hydrates, cells could not function, nor dissolving salts, such as ionic beverages.
“We have made another step in the understanding of how hydrates work and how their structure affects their mobility. This is an important advance, no one else has been able to study these substances, their movement and structure with such precision,” said Jelinek, an internationally renowned expert in the theoretical and experimental study of physical and chemical properties of molecular structures on solid surfaces using scanning microscopes.
The Chinese scientists first prepared different types of clusters, when different numbers of water molecules bound to the atom of sodium. The results of these atomic maneuvres were subsequently examined by scientists using specially modified scanning microscopes. However, a fundamental discovery would not have been possible without a special method, the development of which was greatly influenced by Czech physicists, and published this year by Nature Communications magazine in January.
“The difficulty in studying these clusters is their relatively weak internal connections, which are easily broken by the tip of the microscope. Using a new imaging method, we can overcome this obstacle by hanging a single carbon monoxide molecule on the tip’s apex. Thanks to its presence, we are able to see not only whether the ion surrounds one, two or even three water molecules, but also monitor the arrangement of individual molecules without disrupting the structure of the studied ion hydrate cluster,” Jelinek explained.
When scientists knew the composition and form of the observed clusters in detail, they moved them by electrical pulses and then measured their mobility. In general, the higher the mobility of clusters, the more effective they are. “We found that the sodium ion hydrated with three molecules of water was the fastest. This means that this type of cluster will play an important role in the ability to manage sodium transport in biology or to influence corrosion,” Jelinek added.
The ability to see individual molecules, manipulate them, and even show chemical bonds was unimaginable a few years ago. This has been facilitated by the development of atomic resolution scanning microscopes that have opened up new possibilities for scientists, among other things, in the field of the characterization and modification of nanostructures.
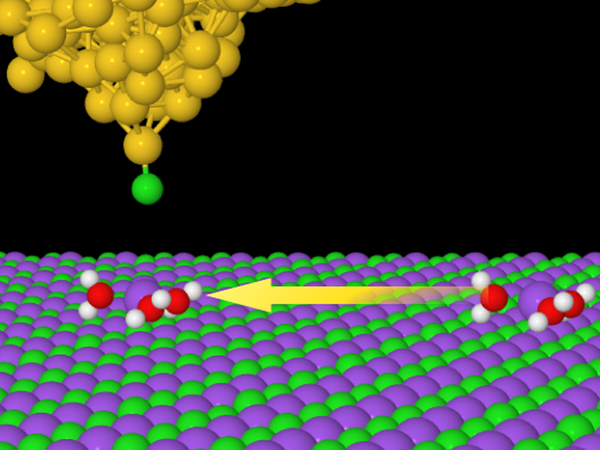
In the picture:
A schematic illustration of the induced movement of the ionic hydrate towards the tip of the microscope by means of an electric pulse. Research has shown that an ionic hydrate consisting of a single sodium atom (purple ball) surrounded by three molecules of water (hydrogen - white ball, oxygen - red ball) shows significantly greater mobility than other types of hydrate formed by a different number of water molecules. This finding demonstrates that the mobility of ion hydrates is greatly influenced by their structure.